CBSE Class 12 Chemistry Exam 2023 Important Questions: The Central Board of Secondary Education is the largest and most popular school board in India, and millions of students are currently enrolled in it. The annual CBSE Exams are an event like no other, and now the 2023 final examinations have arrived. The next paper is of Class 12 Chemistry on 28 February. Chemistry is an important subject in CBSE Class 12 and is essential for both medical and non-medical science stream students. The subject also requires a ton of practice, especially the important topics for class 12 chemistry, like Organic Chemistry and d- and f-block elements. On that note, we bring you the most important topics, chapters and important questions with answers for CBSE Class 12 chemistry exam 2023 to help you with some much-needed last minute revision.
Must Read: CBSE Class 12 Chemistry Sample Paper 2022-23
Must Read: CBSE Class 12 Chemistry Practice Paper 2023
CBSE Class 12 Chemistry Marking Scheme and Blueprint 2023
|
S. No. |
Title |
Marks |
|
1 |
Solutions |
7 |
|
2 |
Electrochemistry |
9 |
|
3 |
Chemical Kinetics |
7 |
|
4 |
d -and f -Block Elements |
7 |
|
5 |
Coordination Compounds |
7 |
|
6 |
Haloalkanes and Haloarenes |
6 |
|
7 |
Alcohols, Phenols and Ethers |
6 |
|
8 |
Aldehydes, Ketones and Carboxylic Acids |
8 |
|
9 |
Amines |
6 |
|
10 |
Biomolecules |
7 |
|
|
Total |
70 |
Why Important Topics and Chapters for Class 12 Chemistry are Beneficial?
Chemistry is no piece of cake. It is a challenging subject that can even surpass physics and maths in difficulty level sometimes due to its conceptual, descriptive and numerical portion. Chemistry requires a deep understanding of the fundamentals, memorisation of several formulas, rules and exceptions, significant theoretical knowledge and calculation skills. All this requires practice but not blind practice. You must know which topics are important and which chapters are frequently asked in the exam.
Related: CBSE Chemistry Previous Year Question Paper Class 12 with Solution
Key Highlights of CBSE Chemistry Important Topics for Board Exam 2023
Organic chemistry is the most important topic for CBSE Class 12 Chemistry exam. It also comes in handy during the JEE and NEET preparation and higher studies. Organic chemistry is complex but becomes very easy with constant practice. D & f-block elements are also important chapters, but unlike organic chemistry, they aren’t so easy to master. It’s necessary for students to solve previous year’s papers, sample papers and important questions to get an idea of the difficulty level of questions and how best to attempt them. We have covered important questions for class 12 Chemistry for all chapters in the following section.
Related: CBSE Class 12 Chemistry Sample Paper 2022-23
Important Questions For Class 12 Chemistry CBSE Board
Section A: MCQs (1 Marks)
1. Which is not a colligative property?
(a) Osmotic pressure
(b) Lowering of vapour pressure
(c) Depression in freezing point
(d) Molal elevation constant
Answer: (d)
2. Negative deviation from Raoult’s law is observed in which one of the following binary liquid mixtures?
(a) Ethanol and acetone
(b) Benzene and toluene
(c) Acetone and chloroform
(d) Chloroethane and bromoethane
Answer: (c)
3. How long would it take to deposit 50 g of Al from an electrolytic cell containing Al2O3 using a current of 105 amperes?
(a) 1.54 h
(b) 1.42 h
(c) 1.32 h
(d) 2.15 h
Answer: (b) 1.42 h
4. A first order reaction takes 40 min for 30% decomposition. t1/2 will be
(a) 77.7 min
(b) 52.5 min
(c) 46.2 min
(d) 22.7 min
Answer: (a)
5. The property which is not characteristic of transition metals is
(a) variable oxidation states.
(b) tendency to form complexes.
(c) formation of coloured compounds.
(d) natural radioactivity.
Ans.(d) natural radioactivity.
6. Lanthanoid contraction is due to increase in
(a) atomic number
(b) effective nuclear charge
(c)atomic radius
(d)valence electrons
Ans. (b)effective nuclear charge
7. When salicylic acid is heated with Zn dust , what will be the main product ?
a) Benzene
b) phenol
c) Toluene
d) Benzoic acid
Ans. (d)
8. Identify the product of the following reaction :
a) Benzaldehyde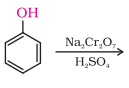
b) Benzene
c) Benzoquinone
d) Benzoic acid
Answer: (c)
Section B: Very Short Answer (2 Marks)
Q. 1 Why a person suffering from high blood pressure is advised to take minimum quantity of common salt ?
Answer: Osmotic pressure is directly proportional to the conc. of solutes. Our body fluid contain
a number of solutes. On taking large amount of salts, ions enter the body fluid there by
raising the conc. of the solutes. As a result osmotic pressure increases which may rapture
the blood cells.
Q. 2 State Kohlrausch law of independent migration of ions. Why does the conductivity of a solution decrease with dilution?
Answer: The limiting molar conductivity of an electrolyte is the sum of the limiting ionic conductivities of the cation and the anion each multiplied with the number of ions present in one formula unit of the electrolyte Λ°m for AxBy = xλ°+ + yλ°– For acetic acid Λ° (CH3COOH) = λ°CH3COO– + λ°H+ Λ°(CH3COOH) = Λ° (CH3COOK) + Λ° (HCl) – Λ° (KCl)
Q.3. (i) State rate law?
(ii) For a reaction in 10 minutes concentration of reactant reduced from 0.12μg to 0.06 μg and in next 10 minutes it becomes 0.03 μg. find the order of this reaction.
Ans. (i) Expression in which reaction rate is given in terms of molar conc. of reactants with each term rise to some power which may or may not be same as the stoichiometric coefficient of the reacting species in a balanced chemical equation.
(ii) Since in every 10 minutes concentration becomes half. Half-life is independent of concentration
for first order reaction. So it is a first order reaction
Q.4 Assertion: Copper metal gets readily corroded in acidic aqueous solution such as HCl and dil. H2SO4 Reason: Free energy change for this process is positive.
Ans: (d)Assertion is wrong statement but reason is correct statement
Non-oxidising acids (HCI and dil. H2SO4) do not have any effect on copper. However, they dissolve the metal in presence of air. As it is a non-spontaneous process so, ΔG cannot be -ve.
5. Why p-Nitro phenol is more acidic than Cresols?
Ans. In substituted phenols, the presence of electron withdrawing groups such as nitro group enhances the acidic strength of phenol. This effect is more pronounced when such a group is present at ortho and para positions. It is due to the effective delocalization of negative charge in phenoxide ion when substituent is at ortho or para position. On the other hand, electron releasing groups, such as alkyl groups, in general, do not favour the formation of phenoxide ion resulting in decrease in acid strength.
Section C: Short Answer (3 Marks)
1. Distinction between molarity and molality.
Ans: Molarity: It is the number of moles of solute dissolved in 1 litre of solution. It is temperature dependent. Molality : It is the number of moles of solute dissolved in 1 kg of the solvent and independent of temperature.
2. Define an ideal solution and write one of its characteristics.
Ans: An ideal solution may be defined as the solution which obeys Raoult’s law exactly over the entire range of temperature and pressure. For ideal solution Heat of mixing is zero Volume change of mixing is zero.
3.Iron and nickel are used to make electrochemical cell by using a salt bridge to join a half cell
containing 1 M Fe2+ (aq) in which a strip of iron has been immersed to a second half cell
which contains 1 M Ni2+ (aq) in which a strip of Ni has been immersed ? A voltmeter is
connected between the two metal strips :
E°Fe²+/Fe = – 0.44 V E°Ni²+/Ni = – 0.25 V
(a)Write the name of the cathode and anode.
(b)Write the half reactions involved ?
(c)What would be the
Ans.
(a) Anode : Fe , Cathode : Ni
(b)Reaction at anode : Fe ———→ Fe2+ + 2 e–
Reaction at cathode : Ni2+ + 2 e– ———→ Ni
(c)Voltmeter reading decreases.
4. Based on the data, arrange Fe2+, Mn2+ and Cr2+ in the increasing order of stability of +2 oxidation state: E°Cr3+/Cr2+ = – 0.4 V, E°Mn3+/Mn2+ = 1.5 V, E°Fe3+/Fe2+ = 0.8 V
Answer: As the value of reduction potential increases, the stability of +2 oxidation state increases. Therefore, correct order of stability is Cr3+ | Cr2+ < Fe3+ | Fe2+ < Mn3+ | Mn2+.
Section D: Case-Based Answer (4 Marks)
- Read the following paragraph and answer the questions:
Colligative properties of a solution depend upon the number of moles of the solute dissolved and do not depend upon the nature of the solute. However, they are applicable only to dilute solutions in which the solutes do not undergo any association or dissociation. For solutes undergoing such changes, Van’t Hoff introduced a factor, called Van’t Hoff factor (i). This has helped not only to explain the abnormal molecular masses of such solutes in the solution but has also helped to calculate the degree of association or dissociation.
(i) What is Van’t Hoff factor (i) for a compound undergoing tertramerization in an organic solvent?
(ii) Arrange the following in the increasing order of freezing point
0.1M Al2(SO4)3, 0.1M KCl, 0.1M Glucose, 0.1M K2SO4
(iii) The molar mass of Sodium Chloride determined by elevation of boiling point method is found to be abnormal. Why?
(iv) What is the elevation of boiling point of a solution of 13.44g of CuCl2 in 1kg of water?
(Kb for water = 0.52Kkg/mol-1, molar mass of CuCl2 = 134.4g/mol)
(v) Equimolal solutions of NaCl and BaCl2 are prepared in water. Freezing pint of NaCl is found to be -20C. What freezing point do you expect for BaCl2 solution?
Answers:
(i) i= ¼ = 0.25
(ii) 0.1M Al2(SO4)3, 0.1M K2SO4, 0.1M KCl, 0.1M Glucose
(iii) Elevation of boiling point is a colligative property. Since Sodium chloride dissociates in the solution we get abnormal molecular mass.
(iv) ΔTb = iKbm
= 3 x 0.52 x 0.1
= 1.56 K
(v) i for NaCl = 2, i for BaCl2 = 3
ΔTf NaCl = 2
ΔTf BaCl2 3
Hence Tf for BaCl2 = -30C
2. Read the given passage and answer the questions 1 to 5 that follow:
“Car battery is the most important type of secondary cell having a lead anode and a grid of Lead packed with PbO2 as cathode. It is also called lead storage battery. It contains 40% solution of sulphuric acid (Density = 1.294 gmL-1) as electrolyte. The battery holds 3.5 L of the acid. During the discharge of the battery, the density of H2SO4 falls to 1.139 gmL-1 (20% H2SO4 by mass)”
1. Write the reaction taking place at the cathode when the battery is in use.
2. How much electricity in terms of Faraday is required to carry out the reduction of one mole of PbO2
3. What is the molarity of sulphuric acid before discharge?
4. Why is lead storage battery considered a secondary cell?
5. Write the products of electrolysis when dilute sulphuric acid is electrolysed using platinum electrodes.
Answers
1. Cathode reaction is
PbO₂ + SO2 + 4H+ + 2e- → 2PbSO4 + 2H₂O
2. 2F
3. Molarity = (% ×10 ×d) ÷ (Molarity of H2SO4) = (40 x 10 x 1.294) ÷ 98 = 5.28 mol L-1
4. It can be recharged again and again.
5. H2 at cathode and O2 at anode.
3. Read the following paragraph and answer the questions.
Isomerism is a phenomenon in which compounds have the same molecular formula but different physical and chemical properties on account of different structures. The two major types of isomerism are structural and stereo isomerism. The structural isomerism is further divided into four types. Linkage, coordination, ionisation and solvate isomerism while the stereo isomerism is divided into two types: Geometrical and optical isomerism.
(a)Name the type of isomerism exhibited by [Co(NH3)3NO2]3
Ans. Linkage isomerism
(b)The complexes [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6]are the examples of which type of isomerism
Ans. Coordination isomerism
(c) Square planar complexes of MX2L2 type with coordination number 4 exhibit geometrical isomerism whereas tetrahedral complexes with similar composition do not .Why?
Ans. Because the relative positions of the ligands attached to the central metal atom are same with respect to each other in tetrahedral complexes.
(d)Name the type of structural isomerism exhibited by the compound [Co(NH3)5SO4Br]
Ans. Ionization isomerism
(e)Write the name of the linkage isomer of [Co(NH3)5NO2Cl2]
Ans. Pentaamminenitrito-O-cobalt(III)chloride
Section E: Long Answer (2 Marks)
1 .a) Explain the following phenomena with the help of Henry’s law.
(i) Painful condition known as bends.
(ii) Feeling of weakness and discomfort in breathing at high altitude.
(b) Why soda water bottle kept at room temperature fizzes on opening?
Answers:
(i) When scuba divers go deep in the sea, solubility of atmospheric gases increases in blood. When the divers come up, there is release of dissolved gases and it leads to the formation of bubbles of nitrogen in our blood capillaries and hence there is painful sensation called bends. To avoid bends; the tanks of scuba divers are filled with He,N₂ and oxygen.
(ii) At high altitude, partial pressure of oxygen is low, it leads to low concentration of oxygen in blood of people living there. Low concentration of oxygen develops anoxia, i.e., unable to think and act properly.
(b) In order to increase the solubility of CO₂ gas in soft drinks and soda water, the bottles are normally sealed under high pressure. Increase in pressure increases the solubility of a gas in a solvent according to Henry’s Law. If the bottle is opened by removing the stopper or seal, the pressure on the surface of the gas will suddenly decrease. This will cause a decrease in the solubility of the gas in the liquid. As a result, it will rush out of the bottle producing a hissing noise or with a fiz.
2 .The rate constant for the first order decomposition of a certain reaction is described by
the equation logk (s-1) = 14.34 – 1.25 x 104 K / T (5)
i) What is the pre-exponential factor and energy of activation for this reaction?
ii) At what temperature will its half-life period be 256min?
Ans:
i) From Arrhenius equation, k = Ae –Ea/RT
log k = log A – Ea / 2.303RT —–(1)
logk(s-1) = 14.34 – 1.25 x 104 K / T —- (2)
Comparing (1) and (2), log A = 14.34, A = Antilog(14.34) = 2.188 x 1014s-1
Ea / 2.303R = 1.25 x 104
Ea = 1.25 x 104 x 2.303 x 8.314 = 239.34kJ/mol
ii) For first order reaction, t ½ = 0.693/k , k = 0.693/ t ½ = (0.693/256 x 60)s-1
log (0.693/256 x 60) = 14.34 – 1.25 x 104 / T
T = 668.96K
3. [i]What happens, when [a] manganate ion reacts with thiosulphate? [b] dichromate ion reacts with iron in presence of acid?
[ii] Explain the following trends in the properties of the members of the transition elements. [a] Mn2+ is more stable than Fe2+ towards oxidation to +3 state. [b] The enthalpy of atomization is lowest for Zn in 3d-seriesof the transition elements. [c] The E0 value for the Mn3+/Mn2+ couples much more positive than that forCr3+/Cr2+ couple.
Ans [i][a] Manganate ion reacts with thiosulphate
8MnO4-[aq]+3S2O32- [aq]+ H2O 8MnO2+ +6SO42- +2OH-
[b] Dichromate ion reacts with iron in the presents of acid
Cr2O72- [aq]+6Fe2+[aq]+14 H+[aq] 2Cr3+ +6Fe3+ +7H2O [ii] [a] Electronic configuration of Mn2+ =[Ar] 3d5 Electronic configuration of Fe2+ =[Ar]3d6 Mn2+ having half filled d orbitals will be more stable than Fe2+ as it has partially filled d orbitals
[b]Zinc has completely filled d orbitals, which limits its tendency to form metallic bonds. Thus it requires least enthalpy to get atomized. [c] Mn3+[3d4] is less stable than Mn2+[3d5] because Mn2+ has stable half filled configuration, Cr3+ has stable 3d3[t2g3] configuration, therefore Cr3+ cannot be reduced toCr2+.
4.What is meant by crystal field splitting? How t2g and eg orbitals are formed in an octahedral complex?
Ans. In case of free metal ion all the five d orbitals have the same energy and are called degenerate orbitals. The five degenerate orbitals of metal ion split into different sets of orbitals having different energies in the presence of electrical field of ligands. This is called crystal field splitting.
In an octahedral complex, the ligands are present at the corners of an octahedron. As lobes of dx2- y2 and dz2 lie along the axes, ie along the ligands the repulsions are more along the axis. The degeneracy of d orbitals is lifted and these split into two set of orbitals t2g and eg, slightly different in energy.
5. How are the following conversions carried out? i) Propene to propan -2-ol ii)Benzyl chloride to Benzyl alcohol iii) anisole to parabromo anisol
Ans:
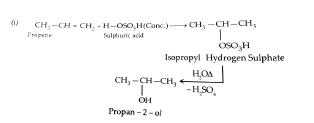
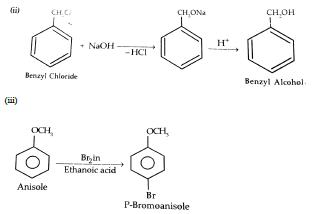
Also Read: CBSE Class 12th Chemistry Notes
CBSE Class 12 Chemistry Deleted Syllabus 2022-23
NCERT Laboratory Manual for CBSE Class 12 Chemistry: Practicals & Projects
Other Important Resources for CBSE Class 12 Exams